

The most frequent serious adverse reactions reported in ≥2% of patients were pneumonia, pyrexia, diarrhea, pneumonitis, pleural effusion, dyspnea, acute kidney injury, infusion-related reaction, musculoskeletal pain, and pulmonary embolism. In Checkmate 743, serious adverse reactions occurred in 54% of patients receiving OPDIVO plus YERVOY. In Checkmate 057, fatal adverse reactions occurred these included events of infection (7 patients, including one case of Pneumocystis jirovecii pneumonia), pulmonary embolism (4 patients), and limbic encephalitis (1 patient). The most frequent serious adverse reactions reported in ≥2% of patients receiving OPDIVO were pneumonia, pulmonary embolism, dyspnea, pyrexia, pleural effusion, pneumonitis, and respiratory failure. In Checkmate 017 and 057, serious adverse reactions occurred in 46% of patients receiving OPDIVO (n=418). Fatal adverse reactions occurred in 7 (2%) patients, and included hepatic toxicity, acute renal failure, sepsis, pneumonitis, diarrhea with hypokalemia, and massive hemoptysis in the setting of thrombocytopenia. In exploratory analyses of patients with PD-L1 expression 2%) serious adverse reactions were pneumonia, diarrhea, febrile neutropenia, anemia, acute kidney injury, musculoskeletal pain, dyspnea, pneumonitis, and respiratory failure. Increased overall survival and improvements in key secondary endpoints observed at four years in the primary population of patients whose tumors express PD-L1 ≥1%
#Checkmate 9la clinical study trial#
chemotherapy with the longest follow-up of any Phase 3 trial for an immunotherapy combination in non-small cell lung cancer
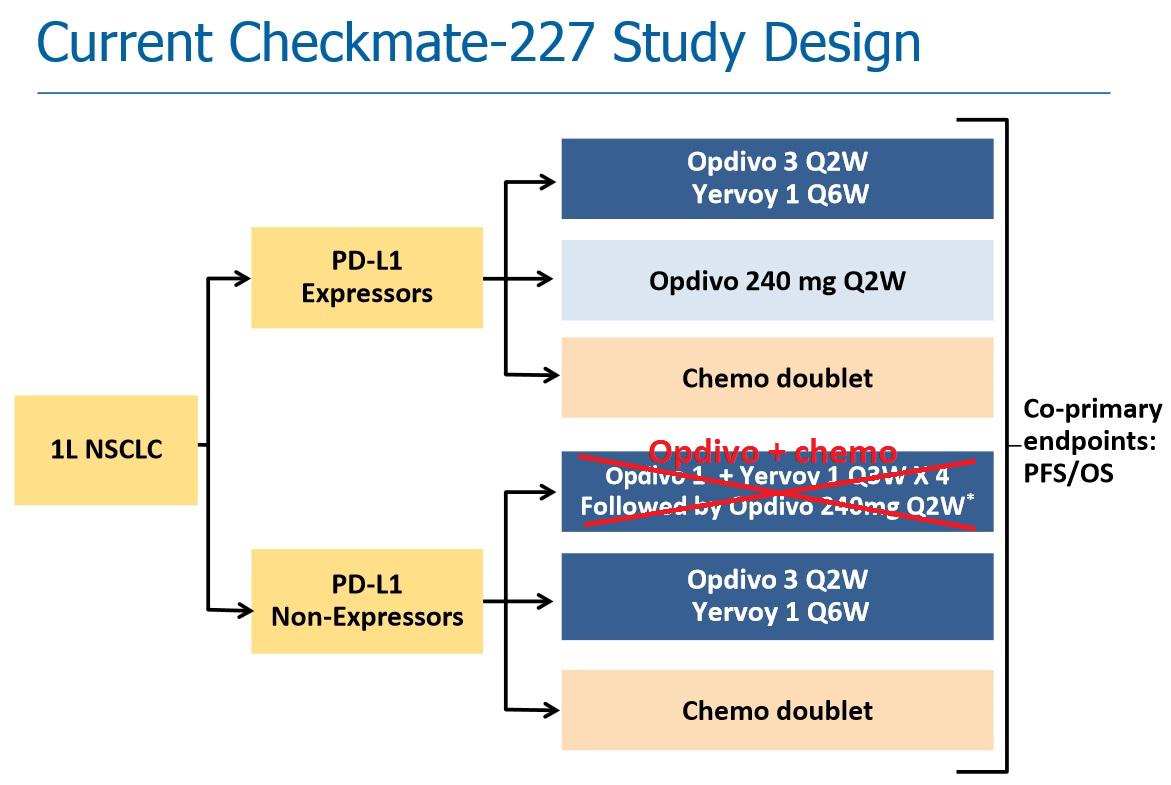
Opdivo plus Yervoy continues to demonstrate a clinically meaningful survival benefit vs.


 0 kommentar(er)
0 kommentar(er)
